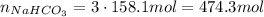Answer:
474.3 mol
Step-by-step explanation:
The reaction between sodium bicarbonate and citric acid may be represented by:
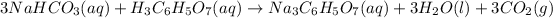
According to the equation, 3 moles of sodium bicarbonate are required to produce 1 mole of sodium citrate. Let's find the number of moles of sodium citrate as our next step. We'll need its mass and its molar mass.
To find the mass of sodium citrate, we may multiply the density by the volume of the product:
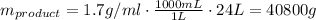
Dividing this by the molar mass of sodium citrate will produce the number of moles:
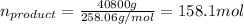
From the stoichiometry of the given equation, we already know that the number of moles of sodium bicarbonate can be found by multiplying the moles of sodium citrate by 3: