Answer:
Delta H is positive and Delta S is negative
Step-by-step explanation:
The expression for the standard change in free energy is:
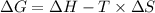
Where,
 is the change in the Gibbs free energy.
is the change in the Gibbs free energy.
T is the absolute temperature. (T in kelvins)
 is the enthalpy change of the reaction.
is the enthalpy change of the reaction.
 is the change in entropy.
is the change in entropy.
For a reaction to be non spontaneous, it means that:-
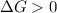
Thus,
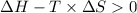
For the above conditions to satisfy,
 must be positive and
must be positive and
 must be negative.
must be negative.