Answer:
1.58x10⁻⁵
2.51x10⁻⁸
0.0126
63.10
Step-by-step explanation:
Phenolphthalein acts like a weak acid, so in aqueous solution, it has an acid form HIn, and the conjugate base In-, and the pH of it can be calculated by the Handerson-Halsebach equation:
pH = pKa + log[In-]/[HIn]
pKa = -logKa, and Ka is the equilibrium constant of the dissociation of the acid. [X] is the concentrantion of X. Thus,
i) pH = 4.9
4.9 = 9.7 + log[In-]/[HIn]
log[In-]/[HIn] = - 4.8
[In-]/[HIn] =
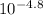
[In-]/[HIn] = 1.58x10⁻⁵
ii) pH = 2.1
2.1 = 9.7 + log[In-]/[HIn]
log[In-]/[HIn] = -7.6
[In-]/[HIn] =
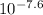
[In-]/[HIn] = 2.51x10⁻⁸
iii) pH = 7.8
7.8 = 9.7 + log[In-]/[HIn]
log[In-]/[HIn] = -1.9
[In-]/[HIn] =
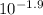
[In-]/[HIn] = 0.0126
iv) pH = 11.5
11.5 = 9.7 + log[In-]/[HIn]
log[In-]/[HIn] = 1.8
[In-]/[HIn] =
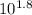
[In-]/[HIn] = 63.10