Answer:
ΔHvap = 431 J/mol
Step-by-step explanation:
The relation between the vapor pressure and the absolute temperature is given by the Clausius-Clapeyron equation.
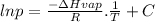
where,
p: vapor pressure
ΔHvap: enthalpy of vaporization
T: absolute temperature
C: constant
We can see that this corresponds to a linear equation with slope -ΔHvap/R and intercept C. If the slope is -3.58 × 10³ K,
-3.58 × 10³ K = -ΔHvap/R
3.58 × 10³ K = ΔHvap/(8.314 J/K.mol)
ΔHvap = 431 J/mol