Answer: The enthalpy change of the reaction is -28.1 kJ/mol
Step-by-step explanation:
To calculate the enthalpy change of the reaction, we use the equation:
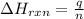
where,
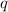 = amount of heat released = -7025 J
= amount of heat released = -7025 J
n = number of moles of metal M = 0.25 moles
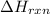 = enthalpy change of the reaction
= enthalpy change of the reaction
Putting values in above equation, we get:
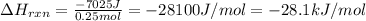
Conversion factor used: 1 kJ = 1000 J
Sign convention of heat:
- When heat is absorbed, the sign of heat is taken to be positive and is written on the reactant side and is considered as an endothermic reaction.
- When heat is released, the sign of heat is taken to be negative and is written on the product side and is considered as an exothermic reaction.
So, the chemical reaction becomes:
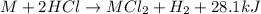
Hence, the enthalpy change of the reaction is -28.1 kJ/mol