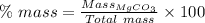Answer:
72.03 %
Step-by-step explanation:
Total mass of dolomite = 9.66 g
Let the mass of Magnesium carbonate = x g
The mass of calcium carbonate = 9.66 - x g
Calculation of the moles of Magnesium carbonate as:-
Molar mass of Magnesium carbonate = 122.44 g/mol
The formula for the calculation of moles is shown below:

Thus,
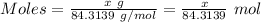
Calculation of the moles of calcium carbonate as:-
Molar mass of calcium carbonate = 100.0869 g/mol
Thus,

According to the reaction shown below:-
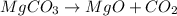

In both the cases, the oxides formed from the carbonates in the 1:1 ratio.
So, Moles of MgO =
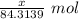
Molar mass of MgO = 40.3044 g/mol
Thus, Mass = Moles*Molar mass =
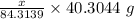
Moles of CaO =
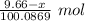
Molar mass of CaO = 56.0774 g/mol
Thus, Mass = Moles*Molar mass =
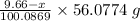
Given that total mass of the oxide = 4.84 g
Thus,
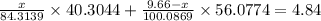
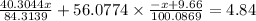
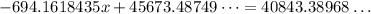
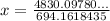
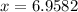
Thus, the mass of Magnesium carbonate = 6.9582 g