Answer:
l = 0
Step-by-step explanation:
Second quantum number, also known as angular momentum quantum number (l), represents the shape of an orbital. Several quantum numbers and their corresponding orbital shapes are worth remembering:
- l = 0, this is an s-shaped orbital;
- l = 1, this is a p-shaped orbital;
- l = 2, this is a d-shaped orbital;
- l = 3, this is a f-shaped orbital
Notice that the angular momentum quantum number might be identified solely from the letter. Since we're considering a
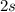 orbital, this means we're dealing with an s orbital. As defined above, s-shaped orbital would have an l value of l = 0.
orbital, this means we're dealing with an s orbital. As defined above, s-shaped orbital would have an l value of l = 0.