Answer:
It will take 7 years ( approx )
Explanation:
Given equation that shows the amount of the substance after t years,
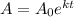
Where,
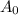 = Initial amount of the substance,
= Initial amount of the substance,
If the half life of the substance is 19 years,
Then if t = 19, amount of the substance =
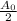 ,
,
i.e.
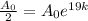
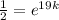
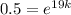
Taking ln both sides,
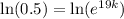
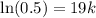
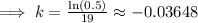
Now, if the substance to decay to 78% of its original amount,
Then
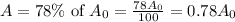
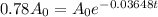
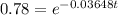
Again taking ln both sides,
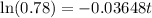
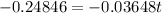
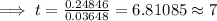
Hence, approximately the substance would be 78% of its initial value after 7 years.