Answer:
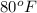
Step-by-step explanation:
We may only compare thermal energy of the two objects when they have identical masses. In this case, this is true: the two objects have equal masses.
The first glass of milk is at a lower temperature, while the second glass of milk is at a higher temperature. Remembering the second law of thermodynamics, heat spontaneously flows from hotter objects to colder ones. This means, the higher the temperature of an object, the greater the thermal energy.
Think about it this way: the higher the temperature, the higher the kinetic energy of the particles (since
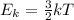 ). The greater the kinetic energy, the greater the velocity. This means a greater amount of energy will be transferred by the object of the same mass but with a higher temperature, as particles are more likely to collide.
). The greater the kinetic energy, the greater the velocity. This means a greater amount of energy will be transferred by the object of the same mass but with a higher temperature, as particles are more likely to collide.