Answer:
If the gas inside a rigid steel vessel (constant volume) is heated in such a way that its temperature, measured in Kelvin degrees, doubles then the internal pressure will increase by a factor of 2.
Step-by-step explanation:
Gay-Lussac's law can be expressed mathematically as follows:
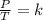
where V = volume, T = temperature, K = Constant
This law indicates that the ratio between pressure and temperature is constant.
This law indicates that, as long as the volume of the container containing the gas is constant, as the temperature increases, the gas molecules move faster. Then the number of shocks against the walls increases, that is, the pressure increases. That is, the gas pressure is directly proportional to its temperature. This must be fulfilled since the relationship must remain constant
In short, when there is a constant volume, as the temperature increases, the gas pressure increases. And when the temperature decreases, gas pressure decreases.
It is desired to study two different states, an initial state and an final state. You have a gas that is at a pressure P1 and at a temperature T1 at the beginning of the experiment. When the temperature varies to a new T2 value, then the pressure will change to P2, and the following will be true:
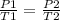
Given the above, it is possible to say that if the gas inside a rigid steel vessel (constant volume) is heated in such a way that its temperature, measured in Kelvin degrees, doubles then the internal pressure will increase by a factor of 2 (doubles too)