Answer:
23.226 psi
Step-by-step explanation:
From the source,
The mass of the carbon dioxide is:- 16.0 g
Molar mass of carbon dioxide = 44.01 g/mol
The formula for the calculation of moles is shown below:
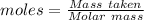
Thus,
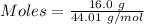
Moles of
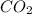 = 0.3636 moles
= 0.3636 moles
Volume = 3.79 L
n = 0.3636 mol
Temperature = 297 K
Using ideal gas equation as:
PV=nRT
where,
P is the pressure
V is the volume
n is the number of moles
T is the temperature
R is Gas constant having value = 0.0821 L.atm/K.mol
Applying the equation as:
P × 3.79 L = 0.3636 mol × 0.0821 L.atm/K.mol × 297 K
⇒P = 2.34 atm
Also, 1 atm = 14.7 psi
So, Pressure =
 psi = 37.926 psi
psi = 37.926 psi
THus, pressure by the gas is:-
P = Total pressure - Atmospheric pressure = 37.926 - 14.7 psi = 23.226 psi
The carbon dioxide in the cartridge inflate a 3.79 L mountain bike tire to 23.226 psi pressure.