Answer:
Ionic, covalent, covalent, ionic, covalent, covalent
Step-by-step explanation:
In order to solve this problem, let's introduce the basic strategy:
- A compound that has a metal in it, would be ionic;
- A compound that only has nonmetals in it, would be covalent.
Since all compounds here are chloride, we only need to take a look at the other element which makes the substance.
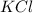 : potassium belongs to group 1A, alkali metals, it's a metal, meaning we have a metallic chloride, this is ionic;
: potassium belongs to group 1A, alkali metals, it's a metal, meaning we have a metallic chloride, this is ionic;
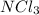 : nitrogen belongs to group 5A, there are only nonmetals present in the group, meaning we have a molecular covalent compound;
: nitrogen belongs to group 5A, there are only nonmetals present in the group, meaning we have a molecular covalent compound;
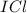 : iodine belongs to group 7A, halogens, they are all nonmetals, this implies we have a covalent compound;
: iodine belongs to group 7A, halogens, they are all nonmetals, this implies we have a covalent compound;
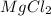 : magnesium belongs to group 2A, alkaline earth metals, it's a metal, meaning we have an ionic compound;
: magnesium belongs to group 2A, alkaline earth metals, it's a metal, meaning we have an ionic compound;
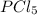 : phosphorus belongs to group 5A, just as nitrogen, it's a nonmetal, meaning we have a covalent compound;
: phosphorus belongs to group 5A, just as nitrogen, it's a nonmetal, meaning we have a covalent compound;
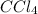 : carbon belongs to group 4A, in this group, we have nonmetals, meaning we have a covalent compound.
: carbon belongs to group 4A, in this group, we have nonmetals, meaning we have a covalent compound.
To summarize, metals (groups 1A, 2A, all elements of group B) would produce ionic compounds, while not having them in your structures would imply that you have a covalent compound.