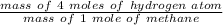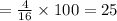Answer:
25
Step-by-step explanation:
In one mole of methane
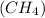 there are 4 moles of hydrogen and one mole of carbon atom.
there are 4 moles of hydrogen and one mole of carbon atom.
Mass of 1 mole of hydrogen atom = 1 g
Mass of 4 moles of hydrogen atom = 4 g
Mass of 1 mole of carbon atom = 12 g
Mass of 1 mole of methane = 12+4 = 16 g
Mass percent of hydrogen in methane =