Answer:
Formula unit mass of
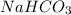 is 84 u.
is 84 u.
Step-by-step explanation:
Formula unit mass can be calculated by the adding individual element mass in the formula of the compound.
The given compound -
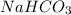
From the given the atomic mass of each element in the given compound is as follows.
Atomic mass of sodium (Na) = 23 u
Atomic mass of Hydrogen (H) = 1 u
Atomic mass of carbon (c) = 12 u
Atomic mass of oxygen (O) = 16 u
The number of atoms in the formula:
1 sodium atom =23 u
1 hydrogen atom = 1 u
1 carbon atom = 12 u
3 oxygen atom = 3 X 16 u = 48 u
_____________________________
Formula mass = 84 u
_______________________________
Therefore, Formula unit mass of
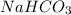 is 84 u.
is 84 u.