Answer:
8
Step-by-step explanation:
Oxidation:
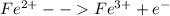
Reduction:
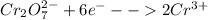
We have to equalise the number of moles of electrons gained and lost in a redox reaction in order to get a balanced reaction.
Hence we have to multiply the oxidation reaction throughout by 6.
and adding the two half-reactions we obtain:

Still the total charge and number of oxygen is not balanced.
Since the reaction takes place in acidic conditions, we will add required number of H+ to the appropriate side to balance the charge and add half the amount of H2O to balance the hydrogen atoms.
We add 14 H+ on LHS and 7H2O on RHS to obtain:
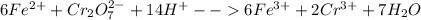
Sum of coefficients of product cations = 6+2 = 8