Answer:
The amount of energy required to transport hydrogen ions from a cell into the stomach is 37.26KJ/mol.
Explanation:
The free change for the process can be written in terms of its equilibrium constant as:
ΔG° =
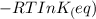
where:
R= universal gas constant
T= temperature
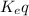 = equilibrum constant for the process
= equilibrum constant for the process
Similarly, free energy change and cell potentia; are related to each other as follows;
ΔG= -nFE°
from above;
F = faraday's constant
n = number of electrons exchanged in the process; and
E = standard cell potential
∴ The amount of energy required for transport of hydrogen ions from a cell into stomach lumen can be calculated as:
ΔG° =
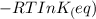
where;
[texK_eq[/tex]=
![([H^+]_(cell))/([H^+(stomach lumen)])](https://img.qammunity.org/2020/formulas/chemistry/college/5f796gcrrhe92r243cxv8uj0nk21cxwifz.png)
For transport of ions to an internal pH of 7.4, the transport taking place can be given as:
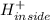 ⇒
⇒
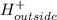
Equilibrum constant for the transport is given as:
![K_(eq)=([H^+]_(outside))/([H^+]_(inside))](https://img.qammunity.org/2020/formulas/chemistry/college/mapg2cwoltvqrjxs8dpbad068o38gh3dp4.png)
![=([H^+]_(cell))/([H^+]_(stomach lumen))](https://img.qammunity.org/2020/formulas/chemistry/college/12ncsvh43wxi90znnrlu2ffxvj3zbzdtrm.png)
![[H^+]_(cell)](https://img.qammunity.org/2020/formulas/chemistry/college/tkpfanj45tmsxygae23xi0u9r9g9nyfaxp.png) = 10⁻⁷⁴
= 10⁻⁷⁴
=3.98 * 10⁻⁸M
![[H^+]_(stomach lumen)](https://img.qammunity.org/2020/formulas/chemistry/college/vy593qbutcj6mjsuxtrjh13k801ayqcgpk.png) = 10⁻²¹
= 10⁻²¹
=7.94 * 10⁻³M
Hence;
![K_(eq)=([H^+]_(cell))/([H^+]_(stomachlumen))](https://img.qammunity.org/2020/formulas/chemistry/college/zgae0i5osgxt298ifi3rcwx5exhcrijlxk.png)
=
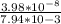
= 5.012 × 10⁻⁶
Furthermore, free energy change for this reaction is related to the equilibrium concentration given as:
ΔG° =
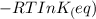
If temperature T= 37° C ; in kelvin
=37° C + 273.15K
=310.15K; and
R-= 8.314 j/mol/k
substituting the values into the equation we have;
ΔG₁ =
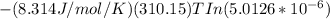
= 31467.93Jmol⁻¹
≅ 31.47KJmol⁻¹
If the potential difference across the cell membrane= 60.0mV.
Energy required to cross the cell membrane will be:
ΔG₂ =
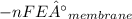
ΔG₂ =
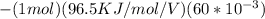
= 5.79KJ
Therefore, for one mole of electron transfer across the membrane; the energy required is 5.79KJmol⁻¹
Now, we can calculate the total amount of energyy required to transport H⁺ ions across the membrane:
Δ
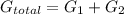
= (31.47+5.79) KJmol⁻¹
= 37.26KJmol⁻¹
We can therefore conclude that;
The amount of energy required to transport ions from cell to stomach lumen is 37.26KJmol⁻¹