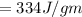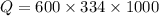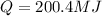Answer:d
Step-by-step explanation:
Given systems are state of matter and do not contain any heat instead Heat is required to change Phase or raise the temperature of the particular system.
For example 600 kg of ice at
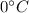
Heat Required to convert it to water at
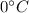 is
is
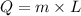
Where L=latent heat of Fusion