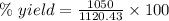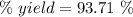Answer:
93.71 %
Step-by-step explanation:
The formula for the calculation of moles is shown below:

For
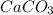 :-
:-
Mass of
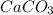 =
=
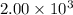 g
g
Molar mass of water = 100.0869 g/mol
The formula for the calculation of moles is shown below:

Thus,
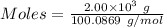
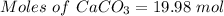
According to the given reaction:
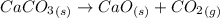
1 mole of
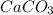 on reaction forms 1 mole of
on reaction forms 1 mole of
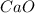
19.98 mole of
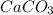 on reaction forms 19.98 mole of
on reaction forms 19.98 mole of
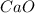
Moles of
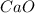 = 19.98 moles
= 19.98 moles
Molar mass of
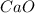 = 56.0774 g/mol
= 56.0774 g/mol
Mass of sodium sulfate = Moles × Molar mass = 19.98 × 56.0774 g = 1120.43 g
The expression for the calculation of the percentage yield for a chemical reaction is shown below as:-
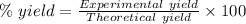
Given , Values from the question:-
Theoretical yield = 1120.43 g
Experimental yield = 1050 g
Applying the values in the above expression as:-