Answer:
Volume occupied by 10 grams of Lithium Oxide at STP is 7.52 L
Step-by-step explanation:
STP (Standard Temperature and Pressure) : It is used while performing calculation on gases and provide standards of measurements while performing experiment.
According to IUPAC (International Union of Pure And applied Chemistry) , standard temperature is 273.15 K and Pressure is 100 kPa (0.9869 atm). At STP condition 1 mole of substance occupies 22.4 L volume .
Molar mass of Lithium Oxide = 29.8 g/mol
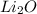 = 2(6.9) + 15.99 = 29.8
= 2(6.9) + 15.99 = 29.8
Mass of 1 mole
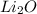 = 29.8 g
= 29.8 g
1 mole
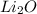 occupies, Volume = 22.4 L
occupies, Volume = 22.4 L
29.8 g
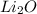 occupies, V = 22.4 L
occupies, V = 22.4 L
1 g
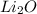 occupies ,V =
occupies ,V =

1 g
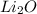 occupies ,V = 0.7516 L
occupies ,V = 0.7516 L
10 g tex]Li_{2}O[/tex] occupies ,V =
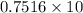 L
L
V = 7.52 L
So, volume occupied by Lithium Oxide At STP is 7.52 L