Answer:
D. All of them would have the same kinetic energy
Step-by-step explanation:
The expression for the kinetic energy of the gas is:-
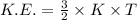
k is Boltzmann's constant =
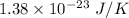
T is the temperature
Since, kinetic energy depends only on the temperature. Thus, at same temperature, at 300 K, all the gases which are
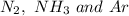 will posses same value of kinetic energy.
will posses same value of kinetic energy.