Answer:
232.9m³ (Option b. is the closest answer)
Step-by-step explanation:
Given:
Air pressure in the lab before the storm, P₁ = 1.1atm
Air volume in the lab before the storm, V₁ = 180m³
Air pressure in the lab during the storm P₂ = 0.85atm
Air volume in the lab before the storm, V₂ = ?
Applying Boyle's law: P₁V₁ = P₂V₂ (at constant temperature)
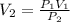
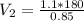
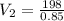
V₂ = 232.9m³
The air volume in the laboratory that would expand in order to make up for the large pressure difference outside is 232.9m³