Answer:
Number of moles nitric acid in the cylinder is 400.539g/mol.
Step-by-step explanation:
From the given,
Weight of empty gas cylinder
![W_(1)= 90.0 lb= 40823.3 grams</p><p>Weight of full cylinder[tex]W_(2) = 116.5 lb= 52843.511 grams</p><p>The critical temperature = 287 K</p><p>The critical pressure 54.3 L</p><p>Molar mass of nitric acid = [tex]M_(NO)](https://img.qammunity.org/2020/formulas/chemistry/college/xj23wtni83ox603ho49w38rulvzt4ckymd.png) = 30.01 g/mol
= 30.01 g/mol
Number of moles nitric acid =
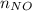 =?
=?
The mass of nitric acid in the cylinder =
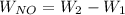
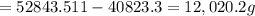
Number of moles of nitric acid =
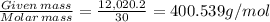
Therefore, number of moles nitric acid in the cylinder is 400.539g/mol.