Answer:
The ration of the molar solubility is 165068.49.
Step-by-step explanation:
The solubility reaction of the magnesium hydroxide in the pure water is as follows.
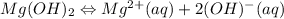
![[Mg^(2+)][OH^(-)]](https://img.qammunity.org/2020/formulas/chemistry/college/x87a2p0ga28ouav7lrw86x7n93ev0a9l7k.png)
Initial 0 0
Equili +S +2S
Final S 2S
![K_(sp)=[Mg^(2+)][OH^(-)]](https://img.qammunity.org/2020/formulas/chemistry/college/77bltn5d8g8h4mirft65lj5zwj75h2s6u0.png)
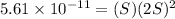
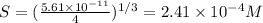
Solubility of
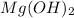 in 0.180 M NaOH is a follows.
in 0.180 M NaOH is a follows.
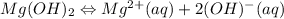
![[Mg^(2+)][OH^(-)]](https://img.qammunity.org/2020/formulas/chemistry/college/x87a2p0ga28ouav7lrw86x7n93ev0a9l7k.png)
Initial 0 0
Equili +S +2S
Final S 2S+0.180M
![K_(sp)=[Mg^(2+)][OH^(-)]](https://img.qammunity.org/2020/formulas/chemistry/college/77bltn5d8g8h4mirft65lj5zwj75h2s6u0.png)
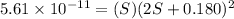
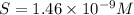
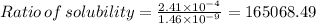
Therefore, The ration of the molar solubility is 165068.49.