Answer:
Supersaturated
Step-by-step explanation:
Let's define the types of solutions in the context of this problem firstly:
- An unsaturated solution is a solution in which addition of more solute would result in dissolution at a given specific temperature. That is, at
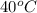 , if we add more than 50 g of
, if we add more than 50 g of
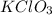 and it still dissolves in 100 g of water, then we have an unsaturated solution.
and it still dissolves in 100 g of water, then we have an unsaturated solution. - A saturated solution is a solution in which we have a maximum amount of a solute that could possibly dissolve in a solvent at a given specific temperature. That is, at
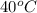 , if we add 50 g of
, if we add 50 g of
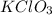 and no more of it dissolves, then we have a saturated solution.
and no more of it dissolves, then we have a saturated solution. - A supersaturated solution is a solution in which we have a greater amount of solute dissolved than we could possibly dissolve under normal circumstances. Let's say that the solubility here is 50 g of
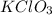 in 100 g of water at
in 100 g of water at
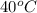 . If we dissolve more than 50 g, then we have a supersaturated solution.
. If we dissolve more than 50 g, then we have a supersaturated solution.
We need to use a solubility curve for salts given below. Notice that the intersection in the y-axis at
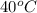 is at about 14 g. This means a saturated solution would be obtained if 14 g of
is at about 14 g. This means a saturated solution would be obtained if 14 g of
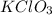 were dissolved in 100 g of water at this temperature. Anything above it would yield a supersaturated solution, below – an unsaturated solution.
were dissolved in 100 g of water at this temperature. Anything above it would yield a supersaturated solution, below – an unsaturated solution.
Hence, we have a supersaturated solution.