Answer:
B)
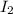 (s) is a stronger oxidizing agent than
(s) is a stronger oxidizing agent than
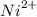 (aq) and Ni(s) is a stronger reducing agent than
(aq) and Ni(s) is a stronger reducing agent than
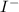 (aq).
(aq).
Step-by-step explanation:
Oxidation is the loss of electrons in a chemical reaction while reduction is the gaining of electrons. The given equations both show a gain of electrons in the forward reaction, however to find the strongest reducing agent we should consider their reduction potentials. The most negative reduction potential indicates a strong reducing agent. In this scenario Ni(s) is a stronger reducing agent than
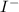 (aq) since it has a reduction potential of -0,26V . The most positive reduction potential indicates a strong oxidizing agent so
(aq) since it has a reduction potential of -0,26V . The most positive reduction potential indicates a strong oxidizing agent so
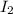 (s) is a stronger oxidizing agent than
(s) is a stronger oxidizing agent than
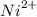 .
.