Answer:
The fraction of original isotope remaining after 12 weeks is 1/8 (0.125)
Step-by-step explanation:
The half life of the radio-isotope = 4 weeks =
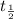
After n half lives, the fraction of original isotope remaining =
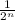
In 12 weeks, the number of half lives = 12/4 = 3 half lives
The fraction of original isotope remaining =
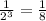
Hence, after 12 weeks, fraction of original isotope remaining is 1/8 (0.125)