Answer:
(a). The work done on the aluminum is -48.6 mJ.
(b). The energy added to it by heat is 16.58 kJ.
(c). The change in its internal energy is 1.658 kJ.
Step-by-step explanation:
Given that,
Mass of block = 1.00 kg
Initial pressure =22.0°C
Final pressure = 40.0°C
(a). We need to calculate the work done on the aluminum
Using formula of work done
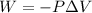
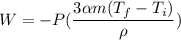
Here,
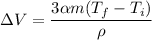
Put the value into the formula
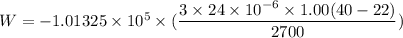
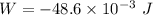
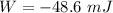
(b). We need to calculate the energy added to it by heat
Using formula of heat
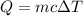
Put the value into the formula
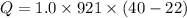
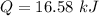
(c). We need to calculate the change in its internal energy
Using formula of internal energy
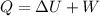
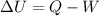
Put the value into the formula
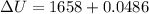
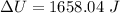

Hence, (a). The work done on the aluminum is -48.6 mJ.
(b). The energy added to it by heat is 16.58 kJ.
(c). The change in its internal energy is 1.658 kJ.