Answer : The correct option is, K is more than 1.
Explanation :
The relation between the equilibrium constant and standard Gibbs free energy is:
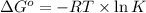
where,
 = standard Gibbs free energy
= standard Gibbs free energy
R = gas constant
T = temperature
K = equilibrium constant
From the above relation we conclude that,
When K > 1 then the value of
 is negative.
is negative.
When K < 1 then the value of
 is positive.
is positive.
When K = 1 then the value of
 is zero.
is zero.
When K = 0 then the value of
 is undefined.
is undefined.
As per question, if the value of ΔG < 0 that means negative value then value of equilibrium constant will be more than 1 that means K > 1.
Hence, the correct option is, K is more than 1.