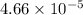Answer: The value of
 is
is
Step-by-step explanation:
We are given:
Initial moles of ammonia = 0.0120 moles
Initial moles of oxygen gas = 0.0170 moles
Volume of the container = 1.00 L
Concentration of a substance is calculated by:
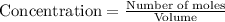
So, concentration of ammonia =
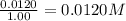
Concentration of oxygen gas =
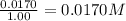
The given chemical equation follows:
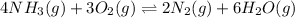
Initial: 0.0120 0.0170
At eqllm: 0.0120-4x 0.0170-3x 2x 6x
We are given:
Equilibrium concentration of nitrogen gas =
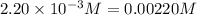
Evaluating the value of 'x', we get:
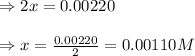
Now, equilibrium concentration of ammonia =
![0.0120-4x=[0.0120-(4* 0.00110)]=0.00760M](https://img.qammunity.org/2020/formulas/chemistry/college/4my0xl4x5k9xa9uea68i2gls0viq35rfm5.png)
Equilibrium concentration of oxygen gas =
![0.0170-3x=[0.0170-(3* 0.00110)]=0.0137M](https://img.qammunity.org/2020/formulas/chemistry/college/s71qqg3ehbm2mu7q96dbfxie87r9gk4ax5.png)
Equilibrium concentration of water =
![6x=[6* 0.00110]=0.00660M](https://img.qammunity.org/2020/formulas/chemistry/college/urv7by3x2fyfsftusw4yf7t29x7miescbh.png)
The expression of
 for the above reaction follows:
for the above reaction follows:
![K_(eq)=([N_2]^2* [H_2O]^6)/([NH_3]^4* [O_2]^3)](https://img.qammunity.org/2020/formulas/chemistry/college/gylcchscle2mha5jifhnxuwkujlpe9iovq.png)
Putting values in above expression, we get:
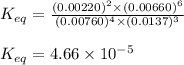
Hence, the value of
 is
is