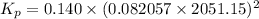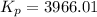Answer:
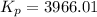
Step-by-step explanation:
The relation between Kp and Kc is given below:
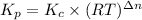
Where,
Kp is the pressure equilibrium constant
Kc is the molar equilibrium constant
R is gas constant
T is the temperature in Kelvins
Δn = (No. of moles of gaseous products)-(No. of moles of gaseous reactants)
For the first equilibrium reaction:
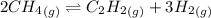
Given: Kc = 0.140
Temperature = 1778 °C
The conversion of T( °C) to T(K) is shown below:
T(K) = T( °C) + 273.15
So,
T = (1778 + 273.15) K = 2051.15 K
R = 0.082057 L atm.mol⁻¹K⁻¹
Δn = (3+1)-(2) = 2
Thus, Kp is: