Step-by-step explanation:
Endothermic reactions are defined as the reactions in which energy of products is more than the energy of the reactants. In these reactions, energy is absorbed by the system. The total enthalpy of the reaction
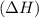 comes out to be positive.
comes out to be positive.
Exothermic reactions are defined as the reactions in which energy of reactants is more than the energy of the products. In these reactions, energy is released by the system. The total enthalpy of the reaction
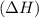 comes out to be negative.
comes out to be negative.
a) Evaporating rubbing alcohol from your skin.
Evaporating is the surface phenomena in which surface molecules of liquid changes into vapor phase. And during the evaporation heat is absorbed by the liquid substance to convert it self into vapor. Hence, endothermic reaction where ∆H° is positive.
Surface molecule of rubbing alcohol absorbs heat and gets evaporated from the skin.
b) Solidifying molten gold into a gold bar
Solidifying is the process in which molecules of liquid changes into solid phase. And during this heat is lost by the liquid substance to convert it into solid phase . Hence, exothermic reaction where ∆H° is negative.
Molten gold loose its energy to change into gold bar.
c) Melting solid gallium metal with the heat from your hand.
Melting is the process in which molecules of solid changes into liquid phase. And during this heat is supplied to the solid substance to convert it into liquid phase. Hence, endothermic reaction where ∆H° is positive .
The heat from our hands get absorbed by solid gallium metal and melts in our hands.