Answer:
121.6 of
 ,Nitrogen is limiting and Hydrogen is in excess.
,Nitrogen is limiting and Hydrogen is in excess.
Explanation:
The reaction involved in this question is known as Haeber's Process and the reaction is as follows
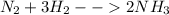
We need to find the moles of each to determine the total product, the excess reagent and limiting reagent.
No. of moles of
 =
=
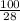 =3.57 mol
=3.57 mol
No. of moles of
 =
=
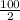 =50 moles
=50 moles
We need moles of
 and
and
 in the ratio of 3:1.
in the ratio of 3:1.
Therefore, we can clearly see that
 is excess. And
is excess. And
 is the limitng reagent.
is the limitng reagent.
As,
 is the limiting reagent a total of 2 * 3.57 moles of
is the limiting reagent a total of 2 * 3.57 moles of
 will be formed.
will be formed.
Therefore, total mass of
 =7.14*17=121.6 g
=7.14*17=121.6 g