Answer:
ΔS = 7.618 J/K
Step-by-step explanation:
The entropy change associated to an isothermal process is:
ΔS =
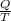
We can calculate Q (heat) how:
Q = nRTln(
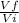 )
)
Where Vf = 2.5v
Vi = v
R = 8.314J/mol.k (Constant of ideal gases)
n: Number of moles = 1 mol
Then
Q = nRTln(2.5)
ΔS =
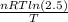
ΔS = (1)(8.314)ln(2.5)
ΔS = 7.618 J/K