Answer:
Ea = 42,0 kJ/mol
Step-by-step explanation:
It is possible to solve this question using Arrhenius formula:

Where:
k1: 3,36x10⁴ M⁻¹ s⁻¹
T1: 344K
Ea = ???
R = 8,314472x10⁻³ kJ/molK
k2 : 7,69 M ⁻¹ s⁻¹
T2: 219K
Solving:
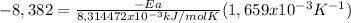
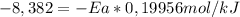
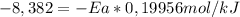
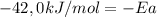
Ea = 42,0 kJ/mol
I hope it helps!