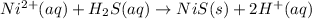Answer:
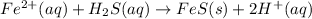
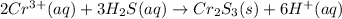
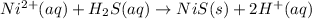
Step-by-step explanation:
We're given the following ions:
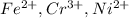
Hydrogen sulfide is a weak acid, so it only ionizes to ions to a very low extent. This means we would expect to see it in a molecular form in a net ionic equation rather than a dissociated form (hydrogen cations and sulfide anions). In each of these net ionic equations, we expect the three cations to displace hydrogen from hydrogen sulfide and form three precipitates.
Firstly, iron(II) displaces hydrogen forming iron(II) sulfide and acidic conditions:
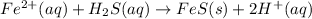
Secondly, chromium(III) cation displaces hydrogen forming chromium(III) sulfide:
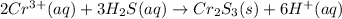
Thirdly, nickel(II) cation displaces hydrogen forming nickel sulfide: