Answer:
trigonal pyramid
Step-by-step explanation:
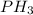
Valence electrons of phosphorus = 5
Valence electrons of hydrogen = 1
The total number of the valence electrons = 5 + 3(1) = 8
The Lewis structure is drawn in such a way that the octet of each atom and duet for the hydrogen in the molecule is complete. So,
The Lewis structure is shown below in the image below.
The central atom , P has 3 bonded pairs of electrons and 1 non bonded.
Number of bond pairs = 3
Number of lone pairs = 1
The formula is
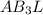 .
.
According to VSEPR theory,
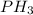 has trigonal pyramidal shape to minimize the repulsions.
has trigonal pyramidal shape to minimize the repulsions.