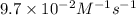Answer:
Step-by-step explanation:
Half life is the amount of time taken by a radioactive material to decay to half of its original value.
Half life for second order kinetics is given by:
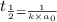
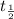 = half life = 15.4 s
= half life = 15.4 s
k = rate constant =?
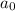 = initial concentration = 0.67
= initial concentration = 0.67
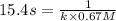
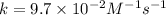
Thus the rate constant for this reaction is