Answer:
All of these
Step-by-step explanation:
In MnO:
mass of Mn = 55 g (as 1 mole of Mn weighs 55 g)
mass of O = 16 g (as 1 mole of O weighs 16 g)
The mass percent of metal Mn=
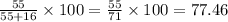
In MnO2:
mass of Mn = 55 g
mass of O = 2*16 = 32 g
The mass percent of metal Mn=
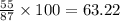
In Mn2O3:
mass of Mn = 2*55 = 110 g
mass of O = 3*16 = 48 g
The mass percent of metal Mn=
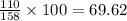
In all the cases the mass percent of metal is greater than 50.