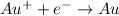Answer:
The half reaction occurring at this electrode will be given as:
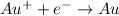
Step-by-step explanation:
In electrochemical cell reduction occurs at anode.
But the gold wire is connected to the positive terminal of the battery which means that gold wire will act as a cathode nad reduction will take place
The half reaction occurring at this electrode will be given as: