Answer:
21.33 g/mol
Step-by-step explanation:
Considering:-
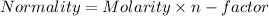
So, Given that:-
Let Molarity of tri-protic acid = x M
n-factor of tri-protic acid = 3 (3 dissociable H)
So,
Normality of tri-protic acid = x/3 N
So, Given that:-
Let Molarity of KOH = 0.400 M
n-factor of KOH = 1 (1 dissociable OH)
So,
Normality of KOH = 0.400 N
Considering:-
At equivalence point
Gram equivalents of acid = Gram equivalents of base
So,
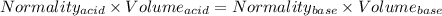
Given that:
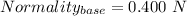
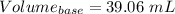
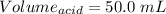
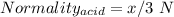
So,
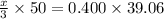
x = 0.93744 M
Also,
Considering:

Or,
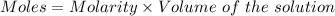
Given :
For tri-protic acid :
Molarity = 0.93744 M
Volume = 50.0 mL
The conversion of mL to L is shown below:
1 mL = 10⁻³ L
Thus, volume = 50.0×10⁻³ L
Thus, moles of tri-protic acid :
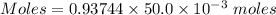
Moles of tri-protic acid = 0.046872 moles
Also, Given mass = 1.00 g
So,
Molar mass = Mass/Moles = 1.00g / 0.046872 moles = 21.33 g/mol
21.33 g/mol is the molecular weight of the unknown acid.