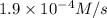The question is incomplete, here is a complete question.
Consider the following reaction between mercury(II) chloride and oxalate ion:
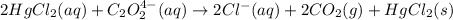
The initial rate of this reaction was determined for several concentrations of
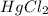 and
and
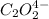 , and the following rate data were obtained for the rate of disappearance of
, and the following rate data were obtained for the rate of disappearance of
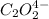 :
:
Experiment
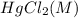
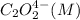 Rate (M/s)
Rate (M/s)
1 0.164 0.15 3.2×10⁻⁵
2 0.164 0.45 2.9×10⁻⁴
3 0.082 0.45 1.4×10⁻⁴
4 0.246 0.15 4.8×10⁻⁵
What is the reaction rate when the concentration of
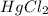 is 0.135 M and that of
is 0.135 M and that of
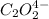 is 0.40 M , if the temperature is the same as that used to obtain the data shown?
is 0.40 M , if the temperature is the same as that used to obtain the data shown?
Answer : The reaction rate will be,
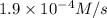
Explanation :
Rate law is defined as the expression which expresses the rate of the reaction in terms of molar concentration of the reactants with each term raised to the power their stoichiometric coefficient of that reactant in the balanced chemical equation.
For the given chemical equation:
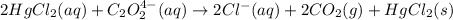
Rate law expression for the reaction:
![\text{Rate}=k[HgCl_2]^a[C_2O_2^(4-)]^b](https://img.qammunity.org/2020/formulas/chemistry/college/xv47vpag04uox3aoumqt6slkwgyu2x1no2.png)
where,
a = order with respect to
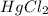
b = order with respect to
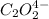
Expression for rate law for first observation:
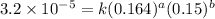 ....(1)
....(1)
Expression for rate law for second observation:
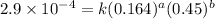 ....(2)
....(2)
Expression for rate law for third observation:
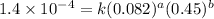 ....(3)
....(3)
Expression for rate law for fourth observation:
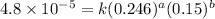 ....(4)
....(4)
Dividing 1 from 2, we get:
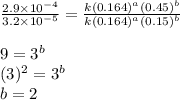
Dividing 3 from 2, we get:
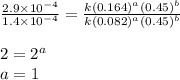
Thus, the rate law becomes:
![\text{Rate}=k[HgCl_2]^1[C_2O_2^(4-)]^2](https://img.qammunity.org/2020/formulas/chemistry/college/5gh5tfsfomppaddqja01xr5pq0qjg6hmpr.png)
Now calculating the value of 'k' by using any expression.
Putting values in above rate law, we get:
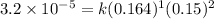
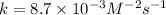
Now we have to determine the reaction rate when the concentration of
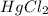 is 0.135 M and that of
is 0.135 M and that of
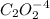 is 0.40 M.
is 0.40 M.
![\text{Rate}=k[HgCl_2]^1[C_2O_2^(4-)]^2](https://img.qammunity.org/2020/formulas/chemistry/college/5gh5tfsfomppaddqja01xr5pq0qjg6hmpr.png)
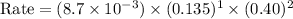
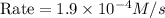
Therefore, the reaction rate will be,