Answer:
The percentage mass of water lost from the total mass of salt is 36%.
Step-by-step explanation:
Molar mass of copper sulfate pentahydrate ,
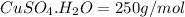
Number of water crystallization = 5
Molar mass of water = 18 g/mol
Percentage of mass water = The percentage mass of water lost from the total mass of salt
Percentage of mass water:
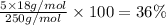
The percentage mass of water lost from the total mass of salt is 36%.