Answer:
0.000121 year⁻¹
Step-by-step explanation:
The half life is defined as the time at which the reactant's concentration reduced to half.
The formula for the half life for a first order kinetic reaction is:
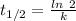
Where,
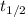 is the half life
is the half life
k is the rate constant.
Thus, Given that:
Half life = 5730 years
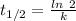
Where, k is rate constant
So,
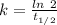
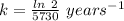
The rate constant or decay constant, k = 0.000121 year⁻¹