Answer:
The correct answer is option c.
Step-by-step explanation:
Alkanes with higher molecular mass has higher boiling point.
Thisis because when the molar mass of the alkanes increases the the surface area increases with which van der Waals forces between the molecules of alkane also increase which increases the association of the molecules of with each other which results in increase in boiling point is observed.
The increasing order of the molar mass of the given alkanes;
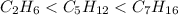
So out of ethane, pentane and heptane . Heptane has highest molecular mass with higher boiling point value. Where as ethane have the lowest value of boiling point.