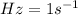Answer:
The energy of a photon of green light with a frequency of is:
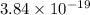 J
J
Step-by-step explanation:
According to Planck's Formula Energy is directly proportional to frequency of photon.
Formula used :
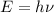
Here h = Planck's constant =
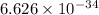 Js
Js
 = frequency of photon =
= frequency of photon =
 Hz
Hz
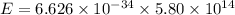
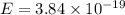 J
J
Note : 1