Answer:
c.
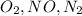
Step-by-step explanation:
We are given that
Atomic mass of nitrogen=14 u
Atomic mass of oxygen=16 u
Molar mass of oxygen gas
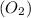 =
=
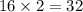 u
u
Molar mass of nitrogen
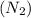 =
=
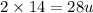
Molar mass of nitric oxide(NO)=16+14=30 u
Translational rms speed=
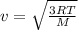
Where R=Gas constant
M=Molar mass
T=Temperature (in K)
Rms speed is inversely proportional to square root of molar mass
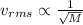
If molar mass is high then translational rms speed is less when the molar mass is less then the translational rms speed is high.
Therefore, the translational rms speed of oxygen is large and translationals rms speed of nitrogen gas is small.
Option c is true