Answer:
65.18% is the percent yield for this reaction.
Step-by-step explanation:

Moles of salicyclic acid =
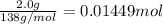
According to reaction 1 mole of salicyclic acid gives 1 mole of aspirin .
Then 0.01449 mole of salicylic acid will give :
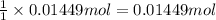 of asprin
of asprin
Mass of 0.01449 moles of aspirin :
= 0.01449 mol × 180 g/mol = 2.6082 g
Theoretical yield of aspirin = 2.6082 g
Experimental yield of aspirin = 1.7 g
The percent yield for this reaction:
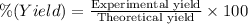
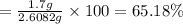
65.18% is the percent yield for this reaction.