Answer : The correct statement is, Alpha particles have a mass number of 4.
Step-by-step explanation:
Alpha particle : It is also known as alpha radiation or alpha ray that consists of 2 protons and 2 neutrons that are bound together into a particle that is identical to the helium nucleus. It is produced in the process of alpha decay.
Alpha decay : In this process, alpha particles is emitted when a heavier nuclei decays into lighter nuclei. The alpha particle released has a charge of +2 units.
The general representation of alpha decay reaction is:
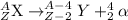
Conversion of proton into neutron leads to releasing in positron.
Positron emission : It is defined as the emission process in which positron particle is emitted. In this process, a proton gets converted to neutron and an electron neutrino particle.
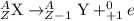
Conversion of neutron into proton leads to release in electron or beta particle.
Beta minus decay : It is a type of decay process, in which a neutrons gets converted to proton, an electron and anti-neutrino. In this the atomic mass number remains same.
The beta minus decay equation is represented as,
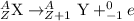
(A is the atomic mass number and Z is the atomic number)
From the given statements we conclude that, the statement alpha particles have a mass number of 4 is correct statement while the other statements are wrong of alpha particles.
Hence, the correct statement is, Alpha particles have a mass number of 4.