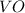Answer : The oxide of vanadium contains the greatest mass percent of the metal is,
Explanation: Given,
Molar mass of vanadium = 50.9 g/mol
Now we have to calculate the mass percent of metal in the following compounds.
For
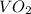 compound :
compound :
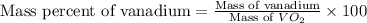
Molar mass of
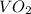 = 82.9 g/mole
= 82.9 g/mole
Now put all the given values in this formula, we get:
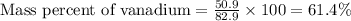
Therefore, the mass percent of vanadium metal is 61.4 %
For
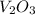 compound :
compound :
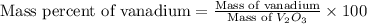
Molar mass of
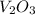 = 149.8 g/mole
= 149.8 g/mole
Now put all the given values in this formula, we get:
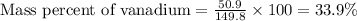
Therefore, the mass percent of vanadium metal is 33.9 %
For
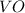 compound :
compound :
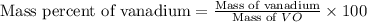
Molar mass of
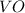 = 66.9 g/mole
= 66.9 g/mole
Now put all the given values in this formula, we get:

Therefore, the mass percent of vanadium metal is 76.1 %
For
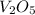 compound :
compound :
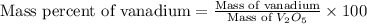
Molar mass of
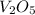 = 181.8 g/mole
= 181.8 g/mole
Now put all the given values in this formula, we get:
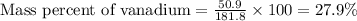
Therefore, the mass percent of vanadium metal is 27.9 %
Hence, the oxide of vanadium contains the greatest mass percent of the metal is,