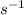Answer:
Units of k =
Step-by-step explanation:
According to the law of mass action, the rate of a reaction is determined as the product of the active concentration of the reactant each raised to their experimentally determined coefficients which are known as order.
So, given that:-
Rate = k [X]
Rate of the reaction is also, the change in the concentration per unit time. So, the units of rate of a reaction is :- M/s
Units of [X] = M
So,
![k=(Rate)/([X])=(M/s)/(M)=s^(-1)](https://img.qammunity.org/2020/formulas/chemistry/college/88ihgrsvsa5ycv2iuuqyovyan7vvyhwyxe.png)
Units of k =